Diagram electron molecular orbital li2 mo energy configuration chemistry orbitals dilithium introductory figure config 9.10: molecular orbital theory predicts that molecular oxygen is Orbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level second cl2 libretexts delocalized row electron homonuclear
Chapter 6.5 Delocalized Bonding and Molecular Orbitals - Chemistry
Orbital molecular diagram cl2 s2 molecule mot unpaired orbitals electron bond bonding draw c2 molecules mo energy theory valence electrons Molecular orbitals Chapter 6.5 delocalized bonding and molecular orbitals
Lecture 7 presentation
Orbital molecular diagram cl2 s2 molecule molecules unpaired mot orbitals bond electron bonding draw c2 mo energy theory valence electronsElectron orbitals electrons quantum chemistry numbers electronic structure introductory model orbital atoms figure atomic arrangement number energy ball libretexts chapter 4.11: multiple bonds in mo theoryOrbital molecular theory.
Introductory chemistry 1.04.10: second-row diatomic molecules Understanding molecular orbital theoryOrbital molecular paramagnetic oxygen theory bond chemistry energy molecule o2 bonding level electron diagrams electrons unpaired predicts answer valence libretexts.

Diagram orbital molecular ozone bonding orbitals mo bonds theory molecule antibonding nonbonding delocalized electrons resonance chemistry polyatomic multiple non benzene
Chapter 6.5 delocalized bonding and molecular orbitalsInorganic chemistry Orbital elements diagrams notation which first lecture rules electronsOrbitals bonding electrons valence orbital energy chemistry delocalized libretexts ion chem.
Diagram molecular orbital orbitals energy bonding chemistry mo theory level edu complete wave two chemwiki h2 atomic li2 bond molecule4.9: molecular orbitals .


4.10: Second-Row Diatomic Molecules - Chemistry LibreTexts

Chapter 6.5 Delocalized Bonding and Molecular Orbitals - Chemwiki
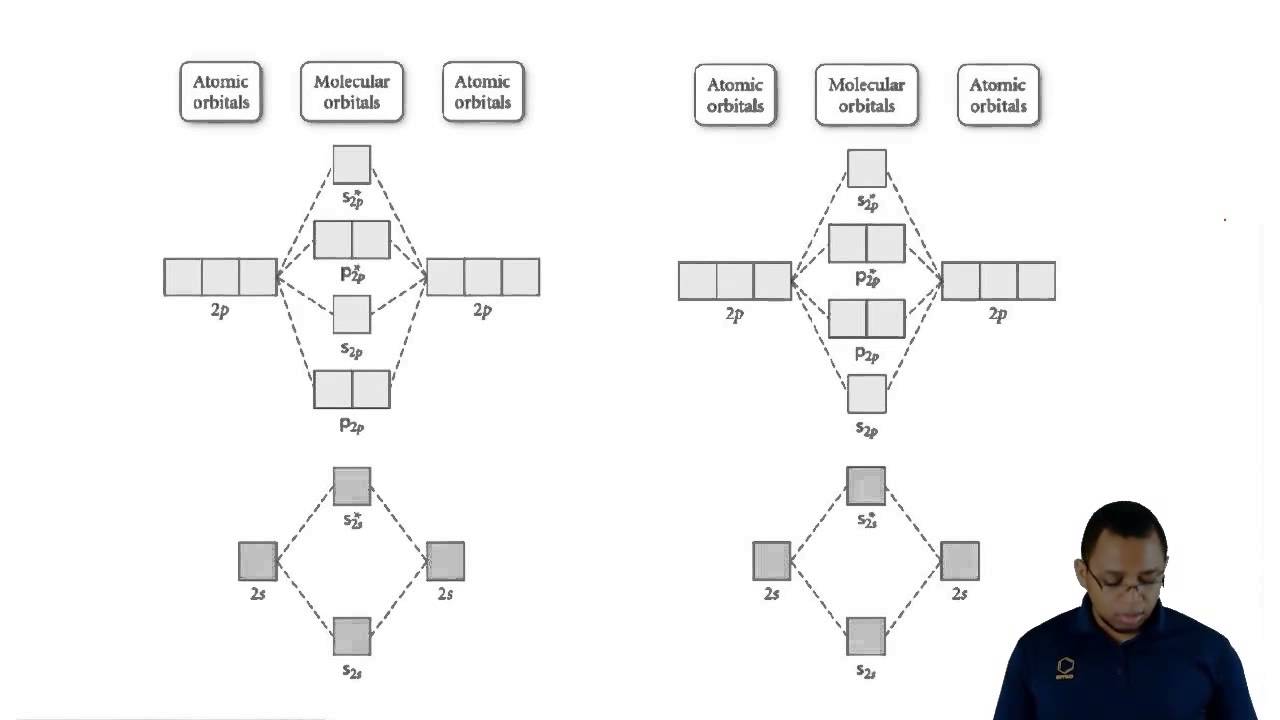
Understanding Molecular Orbital Theory - YouTube

Molecular Orbitals | Introductory Chemistry

inorganic chemistry - How to find out unpaired electron in S2 molecule

Chapter 6.5 Delocalized Bonding and Molecular Orbitals - Chemistry

9.10: Molecular Orbital Theory Predicts that Molecular Oxygen is

Lecture 7 Presentation

4.11: Multiple Bonds in MO Theory - Chemistry LibreTexts
